(SC_CURR) The current number of ARV drug units (bottles) at the end of the reporting period by ARV drug category
Export Indicator
This indicator measures the number of ARV drug units available at the time of reporting. This can serve as an indication of the current stock levels at PEPFAR-supported facilities. The indicator is designed to provide insight into the ‘on-the-shelf’ availability of crucial products, required for HIV treatment.
Data from this indicator may be coupled with SC_ARVDISP to determine how long the quantity of stock will last based on past ARV dispensation records. Similarly, data from this indicator can be used with forecasting data to illustrate that either sufficient stock are available for future or an upcoming need by ARV category exists.
Data from SC_CURR can be used in many ways, such as: (1) to justify a change in the supply plan (i.e., if one ARV drug category is overstocked while another is understocked), (2) to illustrate if a ARV drug category is not being dispensed as anticipated, (3) to determine if an ARV drug category is overstocked, (4) to determine where ARVs may be overstocked, (5) to identify bottlenecks or sites where stock is available and, when coupled with SC_ARVDISP, not dispensed. Data can also be used to examine the relationship between facilties dispensing to patients and sites providing ARVs to dispensing sites (i.e., warehouses) to determine if quantities held at any site are reasonable.
The number of ARV drug units (bottles) at the end of the reporting period by ARV drug category
N/A
How to calculate annual total: This is a snapshot indicator measuring the number of units of ARV drugs currently available at the end of reporting period.
How to collect:
This indicator should be collected from facility dispensing registers or stock records, reported at the site level, based on data available to the facility-based implementing partner, but could include host government-supported Warehouse or Logistics Management Information System(s) (LMIS) as well. Operational Units (OUs) should work with IPs supporting facilities and/or the supply chain IPs to access facility dispensing registers or the LMIS to consolidate dispensing data by site and ARV category for reporting.
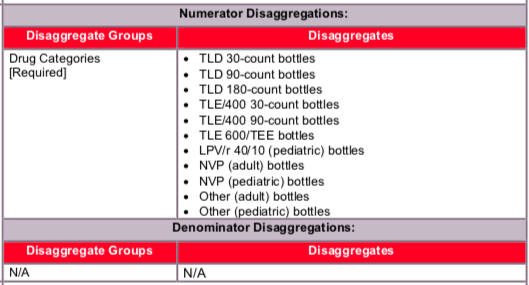
Data should be reported, as indicated, in the categories below:
- TLD 30-count bottles
- TLD 90-count bottles
- TLD 180-count bottles
- TLE/400 30-count bottles
- TLE/400 90-count bottles
- TLE 600/TEE bottles
- LPV/r 40/10 (pediatric) bottles
- NVP (adult) bottles
- NVP (pediatric), (not including NVP 10) bottles
- Other (adult) bottles
- Other (pediatric) bottles
This indicator should be used to describe any anticipated stock-outs, ARV gaps, or are unable to extend their treatment coverage due to supply constraints. In addition, programs should utilize monthly data on each ARV drug category, when available, especially if those data are collected for donor organization and collaboration (such as the PPMR-HIV or SC- FACT).
- If any OU does not support one of the drugs in the disaggregate list, report zero and note it in your narrative.
Do not include PrEP commodities in this indicator.
How to review for data quality: N/A
Reporting level: PEPFAR-supported facilities as well as intermediate or central warehouses and/or locations where ARVs are held in inventory)
Reporting frequency: Semi-Annually
Disaggregate descriptions & definitions:
For drug categories:
- The “Other” categories include medications like single molecule Abacavir or Lopinavir/Ritonavir (stronger than 40/10) which are expected to be a much smaller proportion of the total than Tenofovir-based regimens.
N/A
Indicator changes (MER 2.0 v2.3 to v2.4): New indicator
PEPFAR-support definition:
Nonstandard definition of DSD and TA-SDI:
All facilities that report on TX_CURR (whether DSD or TA_SDI) are required to report on this indicator. Reporting is required regardless of which entity (PEPFAR, Global Fund, host country, etc.) supports the procurement of drugs for the site.
All warehouses that supply drugs to PEPFAR-supported sites are required to report on this indicator.
Guiding narrative questions:
- What data source(s) are used to report on this indicator? Specify whether the data source is: the LMIS, Forecasting software or database, the central medical stores warehouse information system, the PPMR-HIV (Procurement Planning and Monitoring Report for HIV), and/or another source.
- Report when the quantification was done and if the forecast or supply plan have been updated recently, if so provide a date and whether or not the data from SC_CURR informed that action.
- Describe the drug distribution period (e.g., monthly, bi-monthly, etc.)?
- If the SC_CURR data plus an outside forecast or quantification demonstrates that a stock out will occur for any medication at the central or intermediate levels, please describe why and what is being done to mitigate that stock out or if it was planned, i.e., a product no longer recommended in the standard treatment guidelines.
- If the data shows waste, please describe why and what is being done to mitigate this event as well as any plans for environmentally safe destruction. Likewise, if funding is unavailable for destruction, please describe that.
- Are stock-outs a problem at the time of report? Use the data to determine why the stock-out occurred. If data outside SC_CURR and SC_ARVDISP are used to determine why the stock-out occurred, please describe that analysis and actions taken to mitigate.
- During the reporting period, have stock-outs been a problem?
- Use the data to show any anticipated gaps, needed shipments, under- or overstocks, or stock appropriate situations based on current and expected consumption/dispensed to patients.
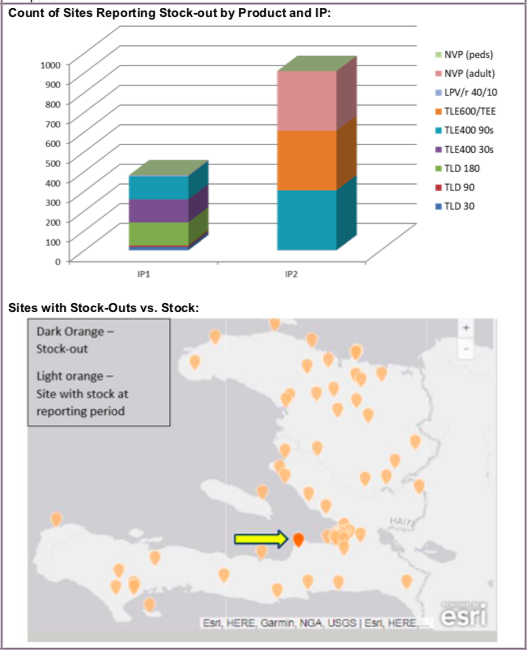
Data Visualization & Use Examples: