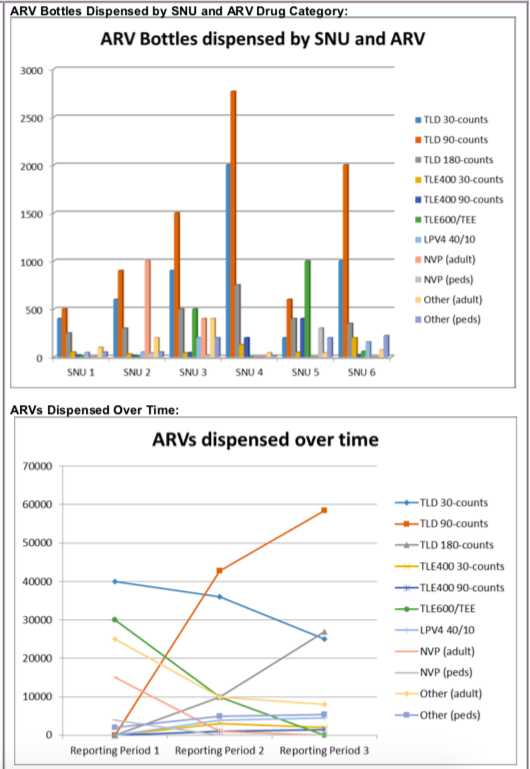
(SC_ARVDISP) The number of adult and pediatric ARV bottles (units) dispensed by ARV drug category at the end of the reporting period
Export Indicator
This indicator measures the number of ARV bottles of several types of ARVs dispensed from a facility. These data should be used to help understand uptake, transition and maintenance of patients to optimized ARV regimens, as well as the phasing out of non- optimal regimens. By reviewing trends over time by each ARV category, programs should monitor coverage of DTG-based regimens relative to other regimens down to the implementing partner and facility level. In addition, data from this indicator should prompt action to investigate any specific sites dispensing regimens which may not be supported by the WHO Standard Treatment Guidelines (STGs).
Number of ARV bottles (units) dispensed within the reporting period by ARV drug category
N/A
How to calculate annual total:
Sum results across reporting periods. This indicator represents the number of ARV bottles dispensed during each reporting period. At Q2, report the total number of bottles dispensed in the first six months of the fiscal year (i.e., Q1 and Q2). At Q4, report the total number of bottles dispensed in the last six months of the fiscal year (i.e., Q3, and Q4).
How to collect:
This indicator should be collected from facility dispensing registers, reported at the facility level, based on data available to the facility-based implementing partner, and could include: host government-supported Logistics Management Information System (LMIS). Operating Units (OUs) should work with IPs supporting facilities and/or the supply chain partners to access the facility dispensing registers or the LMIS to consolidate dispensing data by facility and ARV category.
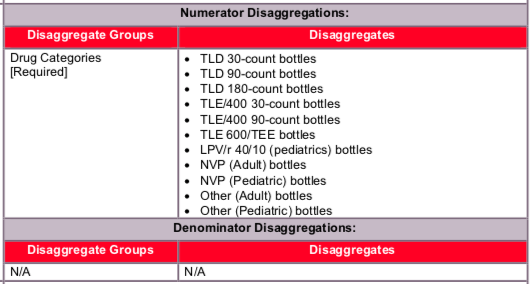
Data should be reported, as indicated, in the categories below:
- TLD 30-count bottles dispensed
- TLD 90-count bottles dispensed
- TLD 180-count bottles dispensed
TLE/400 30-count bottles dispensed
TLE/400 90-count bottles dispensed
TLE 600/TEE bottles dispensed
LPV/r 40/10 (pediatrics) bottles dispensed
NVP (Adult) bottles dispensed
NVP (Pediatric, (not including NVP 10) bottles dispensed
Other (Adult) bottles dispensed
Other (Pediatric) bottles dispensed
This indicator should be reported from PEPFAR-supported facilities which provide treatment or report on treatment indicators, specifically: TX_NEW, TX_CURR, PMTCT_ART, and TB_ART. If an OU or a facility in given OU, does not report on any of these indicators, then they are not required to report on SC_ARVDISP.
If data on ARV dispensation are not available, ‘issues data’ may be used for reporting. ‘Issues data’ is defined as bottles of ARVs provided to facilities from a distribution center. If ‘issues data’ are used for reporting, include the following in the narrative section: (1) an explanation for doing so and (2) what steps will be taken to provide ARV dispensation data in the future. If data on ARV dispensation are incomplete at end of the reporting period, use EITHER ‘issues data’ or ‘dispensed data’. If availability of dispensed data does not align with the PEPFAR reporting period, use the data available from that reporting period and include the following in the narrative: (1) rationale for the data discrepancy and (2) which months are included in the data reported.
If an OU does not support any of the ARV drug categories in the disaggregates list, enter zero for each ARV category and provide an explanation in the narrative.
Do not include any PrEP commodities in this indicator reporting.
How to review for data quality:
At each facility: ensure that the number of drugs dispensed is not greater than the stock issued to the site.
Reporting level: Facility
Reporting frequency: Semi-Annually
Disaggregate descriptions & definitions:
For drug categories:
- “Other” categories includes medications like Abacavir or Lopinavir/Ritonavir (stronger than 40/10). These are expected to be a much smaller proportion of the total than Tenofovir-based regimens
Number of bottles of ARVs by category dispensed to patients
Indicator changes (MER 2.0 v2.3 to v2.4): New indicator
PEPFAR-support definition:
Nonstandard definition of DSD and TA-SDI:
All facilities that report on TX_CURR (whether DSD or TA_SDI) are required to report on this indicator. Reporting is required regardless of which entity (PEPFAR, Global Fund, host country, etc.) supports the procurement of drugs for the facility.
Guiding narrative questions:
- What data source(s) are used for this indicator? Specify whether the quantities reported are those which are dispensed to the patients (preferred) or issued to the facilities from a distribution center.
- Describe data on ARV dispensation data are reported through the system and how orders are calculated?
- Is the system managed through an ‘informed push’? Is it a pull system? Is ARV dispensation data reported actual or is it an average/calculated/estimated?
- If an LMIS is available, how often do facilities report into the LMIS (e.g., monthly, quarterly)?
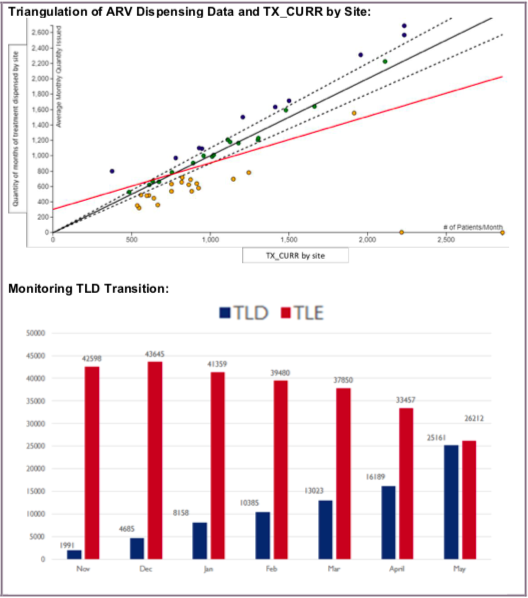
- How does SC_ARVDISP compare to TX_CURR? What is the ratio between the two?
- How do the quantities associated with 90 and 180 count bottles align with multi-month dispensation data?
- If more frequent dispensation data are available (monthly or quarterly LMIS data, for example), especially data from the SC-FACT reporting system (as was recommended in the COP guidance), utilize that to further explain the data reported.
Data Visualization & Use Examples: