(PrEP_NEW) Number of individuals who were newly enrolled on oral antiretroviral pre-exposure prophylaxis (PrEP) to prevent HIV infection in the reporting period
Export Indicator
The indicator measures the ongoing growth of PrEP services. This measure is critical to assess progress in the program’s response to the epidemic in specific geographic areas, and the uptake and utility of PrEP among persons at substantially increased risk of HIV infection.
This indicator permits monitoring trends in PrEP use but does not attempt to distinguish between different modes or regimens of PrEP or to measure the cost, quality, or effectiveness of PrEP provided. These will each vary within and between countries and are liable to change over time.
PrEP has been shown to reduce incident infections among several populations including serodiscordant heterosexual couples, MSM, FSW, and transgender people (TG). The WHO now recommends that oral PrEP containing tenofovir should be offered as an additional prevention choice for people at substantial risk, defined as HIV incidence > 3/100 person- years.
Number of individuals who were newly enrolled on oral antiretroviral pre-exposure prophylaxis (PrEP) to prevent HIV infection in the reporting period
N/A
How to calculate annual total:
Sum results across quarters.
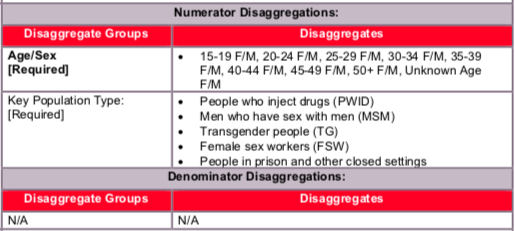
Key Populations (KPs):
Reporting of the key population disaggregation should be consistent with what is described under the KP_PREV “How to review for data quality” section on mutual exclusivity of an individual who falls under multiple KP categories (e.g., FSW who injects drugs). In such instances, the individual should only be reported in ONE KP disaggregation category with which this person is most identified. See Appendix A to support the identification of key populations at service delivery.
The first priority of data collection and reporting of PrEP among key populations must be to do no harm. These data must be managed confidentially to ensure the identities of individuals are protected and to prevent further stigma and discrimination of key populations.
NOTE: In accordance to PrEP guidance, not all PrEP beneficiaries are expected to fall within the KP disaggregates, therefore the total disaggregations for KP does not have to sum to the numerator total. Both KP-specific and clinical partners should complete these KP disaggregations, but only if safe to maintain these files and to report.
Numerator ≥ subtotal of the age/sex disaggregation: The total number people newly enrolled on PrEP (numerator) should be greater or equal to the subtotal of the age/sex disaggregate group.
Reporting level: Facility
Reporting frequency: Semi-Annually
Disaggregate descriptions & definitions:
The numerator is generated by counting the number of people newly enrolled in oral PrEP (including WHO specified regimens “tenofovir-containing PrEP” which could be TDF alone, TDF/FTC, or TDF/3TC) during the reporting period, in accordance with the demonstration project guidance or the nationally approved protocol (or WHO/UNAIDS standards).
N/A
Indicator changes (MER 2.0 v2.3 to v2.4): None
PEPFAR Support definition: