(PrEP_CURR) Number of individuals, inclusive of those newly enrolled, that received oral antiretroviral pre- exposure prophylaxis (PrEP) to prevent HIV during the reporting period
Export Indicator
Tenofovir-containing oral PrEP reduces the risk of HIV acquisition among numerous populations when taken consistently. WHO guidelines recommend offering oral PrEP to those at substantial risk of HIV infection, (incidence rate of 3 per 100 persons per year). This level of elevated risk has been seen among serodiscordant couples with inconsistent condom use when the partner living with HIV is not virally suppressed, men who have sex with men (MSM), transgender people (TG), sex workers (SW) of all genders, and people who inject drugs (PWID), as well as adolescent girls and young women (AGYW) in many parts of sub-Saharan Africa. PEPFAR supports WHO guidelines on the use of PrEP as part of a package of comprehensive structural, biomedical and behavioral prevention services. In most settings, PrEP will be integrated into existing prevention or treatment services for the target population.
As PEPFAR continues to scale up PrEP service delivery, monitoring the PrEP cascade will be important to understand which populations are using this prevention intervention, as well as their length of time using it and their HIV outcome. Understanding the PrEP cascade by population will help improve implementation strategies for those in highest incidence communities initiating PrEP and the retention strategies to support them to stay on PrEP.
Number of individuals that received oral PrEP during the reporting period
N/A
How to calculate annual total:
This is a snapshot indicator. Results are cumulative at each reporting period and should include anyone who received PrEP at ANY TIME during the reporting period. At Q2: report the number of unique individuals that received PrEP in Q1 and Q2. At Q4: report the number of unique individuals that received PrEP in at any point within the fiscal year (i.e., Q1, Q2, Q3, and Q4).
How to collect:
The numerator can be generated by counting the number of individuals that have received PrEP during the reporting period, in accordance with national guidelines or WHO standards, including both those individuals newly initiating on PrEP and those continuing to receive PrEP. PREP_CURR reflects all persons receiving PrEP during the reporting period.
- An individual newly initiating on PrEP will be counted under both PREP_NEW and PREP_CURR during the reporting period.
- Unlike TX_CURR, PrEP_CURR counts the number of individuals that received PrEP at ANY point during the reporting period, so the client does not have to be active on PrEP on the last day of the reporting period like TX_CURR. Unlike HIV treatment, a client does not have to remain on PrEP for the duration of their life. Use of PrEP may cease once an individual is no longer at risk for HIV. This indicator intends to measure client demand for PrEP at any point within the reporting period.
- At Q2: report the number of unique individuals that received PrEP in Q1 and Q2. At Q4: report the number of unique individuals that received PrEP in at any point within the fiscal year (i.e., Q1, Q2, Q3, and Q4).
- If an individual tests positive at his or her three-month PrEP follow-up appointment and is then initiated on PEPFAR-supported treatment in the same reporting period, that individual could be counted as PREP_CURR in addition to TX_NEW and TX_CURR (given successful transfer into the ART program) within that reporting period. They would not be counted under PREP_CURR in subsequent reporting periods.
- The reporting level for this indicator is the facility level only. If PrEP is being provided at community-based sites, these sites should be connected to or have a relationship to a clinical facility. The community sites providing PrEP programming should count the number of individuals currently on PrEP being served through the community service delivery point, and then those data should be reported through the facility connected to that community site.
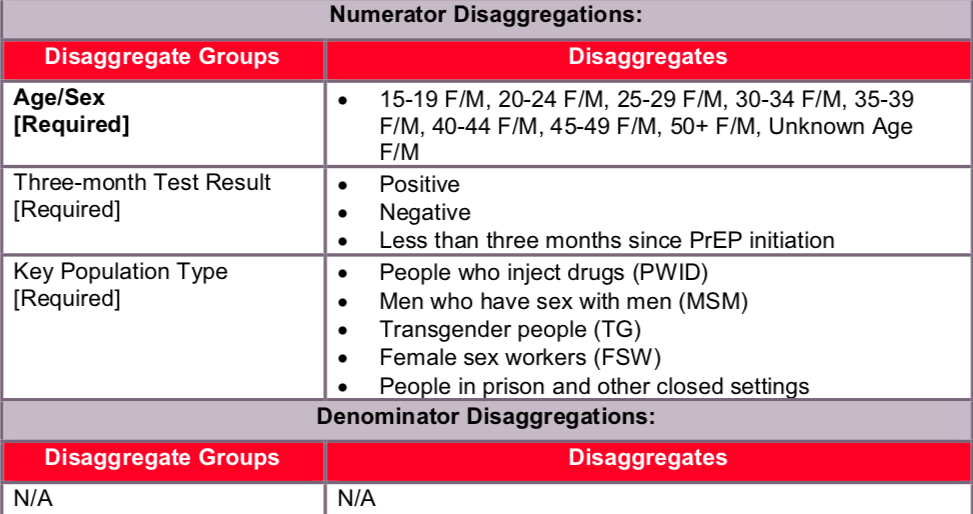
Key populations (KPs):
Reporting of the key population disaggregation should be consistent with what is described under the KP_PREV “How to review for data quality” section on mutual exclusivity of an individual who falls under multiple KP categories (e.g., FSW who injects drugs). In such instances, the individual should only be reported in ONE KP disaggregation category with which this person is most identified. See Appendix A to support the identification of key populations at service delivery.
The first priority of data collection and reporting of PrEP among key populations must be to do no harm. These data must be managed confidentially to ensure the identities of individuals are protected and to prevent further stigma and discrimination of key populations.
NOTE: In accordance with PrEP guidance, not all PrEP beneficiaries are expected to fall within the KP disaggregates, therefore the total disaggregations for KP does not have to sum to the numerator total. Both KP-specific and clinical partners should complete these KP disaggregations, but only if safe to maintain these files and to report.
How to review for data quality:
Numerator ≥ PREP_NEW numerator for the same reporting period (quarter). Numerator ≥ subtotal of three-month test result disaggregate group. Numerator ≥ subtotal of KP population type disaggregate group.
Reporting level: Facility
Reporting frequency: Semi-Anually
Disaggregate descriptions and definitions:
- Age is defined as the age at the time of the visit during the reporting period.
- Three-Month Test is defined as the HIV testing result received by those individuals who present for their three-month follow-up PrEP visit.
- There is also a disaggregate within the indicator to record the result for those individuals who take an HIV test when they were initiated on PrEP less than three months previously (positive/negative/less than three months since PrEP initiation).
- For comprehensive clinical monitoring patients should be receiving two tests within each six-month reporting window.
N/A
N/A
Indicator changes (MER 2.0 v2.3 to v2.4): None
PEPFAR-support definition:
Standard definition of DSD and TA used
Provision of key staff or commodities for PrEP services includes: ongoing procurement of critical commodities (excluding HTS commodities) such “tenofovir-containing PrEP” which could be TDF alone, TDF/FTC, or TDF/3TC or funding for salaries of personnel providing any of the prevention package components (i.e., clinicians, outreach workers, program managers). Staff responsible for the completeness and quality of routine patient records (paper or electronic) can be counted here; however, staff who exclusively fulfill MOH and donor reporting requirements cannot be counted.
Ongoing support for HIV prevention among PrEP services includes: mentoring and supportive supervision; training; organizational strengthening; QA/QI; program design like development of training curricula, PrEP guidance development, or standard operating procedures (SOPs) and follow-up to ensure quality of care; regular assistance with monitoring and evaluation functions and data quality assessments; or supply chain management
Guiding narrative questions:
- What support does PEPFAR provide at this site in terms of staffing, commodities and laboratory services?
- How are you tracking and/or finding individuals who have discontinued PrEP?
- What reasons are individuals citing for discontinuing their use of PrEP?
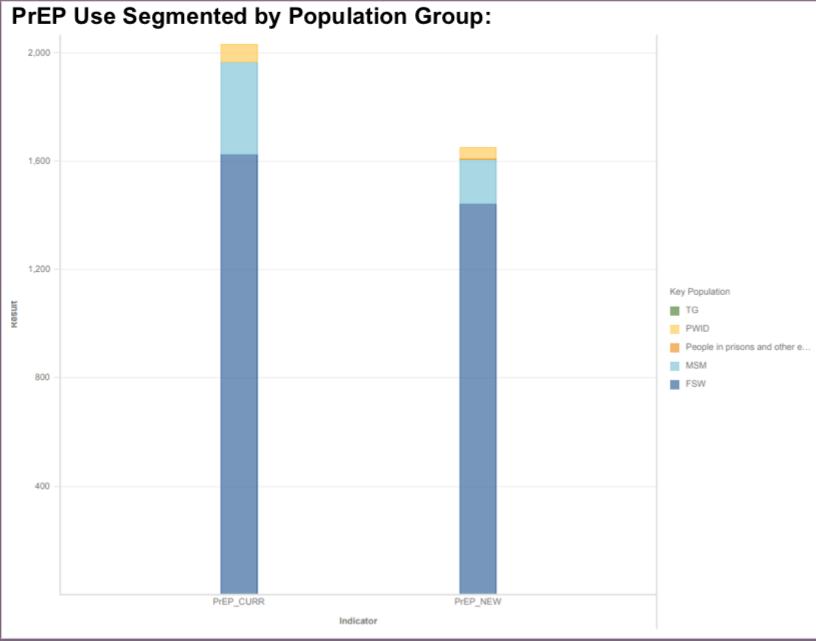
Data Visualization & Use Examples:
Related Indicators
3.15 People who received PrEP, Global AIDS Monitoring 2020: Indicators for monitoring the 2016 Political Declaration on Ending AIDS (https://www.unaids.org/sites/default/files/media_asset/global-aids-monitoring_en.pdf).