(PMTCT_HEI_POS) Number of HIV-infected infants identified in the reporting period, whose diagnostic sample was collected by 12 months of age
Export Indicator
This indicator measures how many HIV-infected infants are identified in a reporting period, disaggregated by age at sample collection and ART initiation status. Identification is by virologic HIV testing: DNA PCR testing of dried blood spots (DBS) or point of care (POC) (e.g., Alere, Xpert) virologic testing. Infants are defined as a child aged between 0 days (newborn) and 12 months of age, and age disaggregation is based on the infant age at the time of sample collection. The infant age reported should not be based on how old the infant was when the result was available to the site but when the sample was collected.
This indicator can include infants identified as HIV-infected on any virologic test by 12 months of age and is not limited to infants identified as HIV-infected on their first virologic test. Infants may be HIV-uninfected on their first virologic test, but at a later age be identified as HIV-infected, and they should be counted in this indicator as long as they were aged 0 - 12 months at the time of subsequent sample collection. Confirmatory testing (collection of a second sample for repeat virologic testing after the first virologic test is positive) is excluded.
Positive Infants and Linkage to ART: PMTCT_HEI_POS will be used to track how many positive infants are identified in a reporting period, and the “ART initiation confirmed” disaggregate can be compared to PMTCT_HEI_POS positive infants to describe rates of linkage to ART for HIV-infected infants (PMTCT_HEI_POS_ART / PMTCT_HEI_POS). The age disaggregate will also help describe linkage rates for very young infants (0-2mo). The proportion of positive infants confirmed as initiating ART can be used to help identify sites with potential successes or challenges in documentation, linkage, and/or initiation of infected infants.
Comparison to TX_NEW age <1: the disaggregate for PMTCT_HEI_POS infants confirmed as initiating ART (sum of 0-2 and 2-12 months) could be compared to “infants <1-year-old initiated on ART (TX_NEW <1)." However, equal values for PMTCT_HEI_POS_ART and TX_NEW age <1 may not be expected, as each indicator may not be counting the same infants. The ART initiation disaggregate within HEI_POS will allow us to report a linked infant ART initiation outcome for each positive infant reported. For more information, see section on "How to review for data quality."
Proxy positivity: When quarterly time period results are aggregated, PMTCT_HEI_POS (numerator) may be able to be compared to PMTCT_EID (numerator) for a proxy positivity calculation. This comparison will provide a poor proxy for positivity for sites or areas with a high percent of test results that are unknown. Combining quarters of data for both PMTCT_HEI_POS and PMTCT_EID for this comparison may reduce the portion of test results that are unknown, especially for infants whose sample was collected near the end of a reporting period. It is also important to note that infants reported under HEI_POS will not be exactly the same as infants reported through PMTCT_EID in the quarterly time period for several reasons: 1) PMTCT_EID is limited to first virologic tests whereas HEI_POS reports infants identified on a first or subsequent test 2) PMTCT_EID is limited to infants with a first virologic test sample collected during the reporting period; whereas PMTCT_HEI_POS includes infants whose positive diagnosis was established during the reporting period, but their sample could have been collected in the prior period.
Birth cohort monitoring: HIV status of infants at the end of the breastfeeding period and the outcomes of the PMTCT program are measured in the PMTCT Final Outcome indicator, PMTCT_FO.
This indicator reports HIV-infected infants identified by virologic HIV testing on any sample collected by 12 months of age: DNA PCR testing of dried blood spots (DBS) or point of care (POC) (e.g., Alere, Xpert) virologic testing.
Limitations and Considerations:
- This indicator does not collect infants with a negative virologic test result or the number of infants whose test result is unknown. As such, “percent unknown” cannot be calculated through the MER indicator, though it is still an important metric for program monitoring. Notifying caregivers of infant test results remains important.
- The infants reported as tested under the revised PMTCT_EID indicator are not necessarily the same infants whose positive results would be reported under the new HEI_POS indicator. Dividing HEI_POS by PMTCT_EID will not provide a precise measure of positivity; however, a proxy positivity could be calculated over a longer time period. See “How to Review for Data Quality” for more information.
Number of HIV-infected infants identified in the reporting period, whose diagnostic sample was collected by 12 months of age.
N/A
How to calculate annual total: Sum results across quarters
How to collect:
This indicator should be collected from the clinical source (i.e., HIV-exposed infant registers or patient records) to ensure unduplicated patient counting and patient care. HIV-exposed infant registers should be used to count HIV-infected infants whose results were returned in the reporting period and the age at the time of sample collection. (If available, information could come from electronic systems). If the standard report does not contain all the required information, individual patient files should be used. Additional supporting information for this indicator can be obtained from standard laboratory information systems (i.e., DNA PCR or POC/near POC log books or electronic systems) however, it will be important to ensure that repeat tests of the same sample or HIV-infected infants receiving a confirmatory virologic HIV test result are not counted twice. Please note that PMTCT_HEI_POS should include all HIV-positive infants identified at the facility in the quarter, regardless of entry point (i.e., not just those identified through the PMTCT entry point). Therefore, a PMTCT clinic may need to compile testing data from other entry points at the facility (e.g., inpatient wards, malnutrition program) to report accurately and completely on this indicator.
Only HIV-infected infants identified as infected by a virologic HIV test on a sample collected when they were between ages 0 through 12 months should be included in this indicator. Infants who initially were identified negative from a first virologic test but who were later identified as HIV-infected after a later virologic test should be included, as long as the infant was still aged 12 months or less at the time of sample collection. Currently, the most commonly used form of virologic testing or nucleic acid testing (“NAT”) is HIV DNA PCR on dried blood spots (DBS) but this indicator also includes HIV-infected infants identified through POC testing (e.g., Alere, Xpert). Serologic testing or “rapid” testing cannot diagnose HIV infection in infant and so infants with a positive serologic test result and either no virologic test result or a negative virologic test result should not be included; however, infants with a positive serologic test and a positive virologic test result should be included.
The numerator is divided into HIV-infected infants who had their diagnostic sample collected for virologic testing between birth and 2 months of age and those whose diagnostic sample was collected between 2 and 12 months of age. The 0- ≤2 month and 2- 12-month time periods are based on age at sample collection for virologic HIV testing, not on date of result available to the facility or caregiver. HIV-infected infants should be reported in the quarterly time period in which they are identified, even if the sample was collected/sent in the previous quarter; their age should be reported by age at the time of collection of the sample that produced the positive result, and not the age when the result was available to the site.
Example scenario to clarify time period and age: an infant has a DBS collected in quarter 3, aged 11 months. Due to long turnaround times, the positive result returns to the site in quarter 4 and staff now identify him/her as HIV-infected at 13 months old. This infant should be counted in quarter 4 as HIV-infected, and his/her age should be reported as 11 months (2-12mo age band).
ART initiation: An additional disaggregate of the numerator is that the HIV positive infant is confirmed as having initiated ART. An HIV-infected infant reported as “ART initiation confirmed” should have documentation of an ART regimen in their record. An HIV-infected infant whose record includes documentation of “referred to ART” or an ART clinic number without evidence of receipt of an ART regimen should not be reported as “ART initiation confirmed.” ART does not include infant ARV prophylaxis regimens for PMTCT.
How to review for data quality:
Linkage and ART Initiation:
- Compare the PMTCT_HEI_POS ART initiation confirmed (disaggregate) to the PMTCT_ HEI_POS numerator to calculate linkage to ART. Significantly <100% or >100% linkage of HIV-infected infants to ART may reflect referrals to different sites, program weakness, or poor data quality and requires review to confirm.
- TX_NEW comparison: HEI_POS_ART disaggregate is expected to be close in value to TX_NEW age <1; however, some discrepancies could be expected and significant discrepancies should be reviewed to confirm. These values may differ in part because the age disaggregate definitions for these indicators differs. TX_NEW age is based on age at ART initiation, while PMTCT_HEI_POS is based on age at virologic sample collection. Scenario: An infant’s virologic sample was collected when the infant was 11 months old near the end of Q1. The infant’s positive result was available to the site in Q2 and she started ART in Q2 at 13 months of age. Under PMTCT_HEI_POS in Q2, she would be reported as “Positive, ART initiation confirmed, age 2-12mo;” however, under TX_NEW in Q2 she would be reported in the 1-9-year age group.
Proxy positivity: it is useful to review proxy positivity (PMTCT_HEI_POS / PMTCT_EID) across sites or locations to identify potential outliers for further review. Summing multiple quarters of data is recommended, as quarter-specific comparisons may provide a less accurate proxy. See “How to use” section for more considerations.
Reporting level: Facility
Reporting frequency: Quarterly
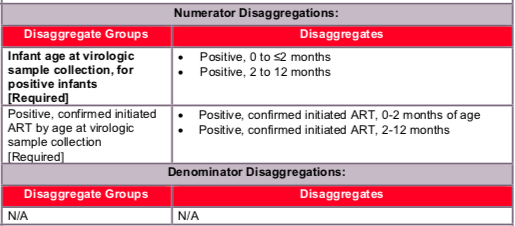
Disaggregate descriptions and definitions:
Description of infant age at virologic sample collection for positive infants: For the numerator to be calculated, implementing partners are required to report:
- HIV-infected infants identified in a quarter, disaggregated by the age at time of sample collection: 0-2 months of age, or between 2-12 months of age. These values will auto-sum to the numerator.
Description of positive, confirmed initiated ART by age at virologic sample collection:
- Implementing partners are required to note HIV positive infants, disaggregated by age 0-≤2months and between 2-12 months, who are confirmed as initiating ART by:
- Positive, confirmed ART initiation, infant was between 0-2 months of age at age time of virologic sample collection
- Positive, confirmed ART initiation, infant was between 2-12 months of age at time of virologic sample collection
This indicator excludes confirmatory testing. It includes 2 required sets of disaggregations: 1) disaggregation by age for positive infants based on the infant’s age at specimen collection for virologic testing; 2) Confirmation of ART initiation, also disaggregated by age at specimen collection.
Indicator changes (MER 2.0 v2.3 to v2.4): None
PEPFAR-support definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits (e.g., including but not limited to DBS bundles or collection kit, POC/near POC sample collection kits and testing devices), ARVs including infant prophylaxis, lab commodities; or funding for salaries of health care workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT service providers, clinical mentoring and supportive supervision of PTMCT service sites, infrastructure/renovation of facilities, support for PMTCT service data collection, reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting and supply management, support of lab clinical monitoring of patients, supporting patient follow- up/retention, support of mother mentoring programs.
Guiding narrative questions:
- Describe the data source used for reporting on this indicator, and any key information about data quality that is important for interpretation of quantitative results.
- Linkage: (PMTCT_HEI_POS confirmed initiated ART (disaggregation) / PMTCT_HEI_POS total numerator). Please describe rates of linkage of positive infants (including young infants, ages 0-2 based on age of virologic sample collection) by subnational area. Please provide context for areas with low linkage rates, and describe activities aimed at improving infant ART initiation.