(PMTCT_ART) Percentage of HIV-positive pregnant women who received ART to reduce the risk of mother-to-child-transmission (MTCT) during pregnancy
Export Indicator
Track progress toward ensuring that all pregnant women who attend PEPFAR-supported antenatal care (ANC) know their HIV status and are initiated on ART.
Number of HIV-positive pregnant women who received ART to reduce the risk of mother-to-child-transmission during pregnancy
PMTCT_STAT_POS (see PMTCT_STAT)
How to calculate annual total: Sum results across quarters for both the numerator and denominator.
How to collect:
Data source is the ANC or PMTCT register depending on country context (in many high HIV prevalence settings information on the number of women receiving ART regimens is integrated into the ANC register). There is a risk of double counting, as a pregnant woman receiving ART at ANC should have multiple visits for each pregnancy. Therefore partners should ensure a data collection and reporting system is in place to minimize double counting of the same pregnant woman across visits including a paper based longitudinal ANC or PMTCT register (meaning a register that is able to record all information about 1 pregnancy in one location, with rows or columns that allow for recording information on multiple visits during that pregnancy) or an electronic medical record/patient tracking system. There is also a risk of undercounting if those women who are already on ART prior to attending ANC are not documented, therefore the ANC register should document both “New on ART” and “Already on ART at the beginning of the current pregnancy”.
Note: Those women reported in PMTCT_ART including newly enrolled on ART and already on ART at the beginning of pregnancy should also be reported in the TX_NEW and TX_CURR indicators, respectively. Women who are already on ART should not be counted in TX_NEW. PMTCT_ART is about initiation of ART (yes/no) or already on ART (yes/no). This will most likely be captured at ANC1 but may be captured at a future ANC visit. Women initiated on ART during L&D or breastfeeding should not be reported under PMTCT_ART but should still be reported under TX_NEW.
How to review for data quality:
Review any site with over 100% coverage or very low coverage to ensure they reflect expected results. In general, services should be reported at the site where they are delivered (however PMTCT_ART- “already on treatment” and PMTCT_STAT_POS “known positive at entry” are exceptions, see details under description of disaggregate below). Therefore, coverage at site level must be understood within the context of the service delivery model at that site. For example, in local areas where ART is integrated into ANC and low volume PMTCT sites are only testing for HIV and then referring women to other facilities for ART, the expectation is that for one individual PMTCT_STAT_POS (newly tested) will be documented at one facility and PMTCT_ART (new on ART) would be documented at another facility leading to the appearance of greater than >100% coverage at one site and 0% coverage at another.
Reporting Level: Facility
Reporting frequency: Quarterly
![]()
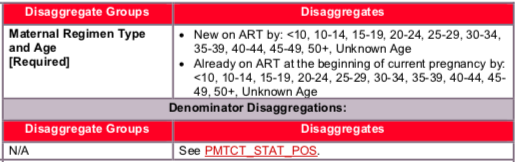
- The number of HIV-positive pregnant women newly initiated on ART should only be counted in a regimen category if she actually received the regimen. Referral alone for ART should not be counted. Additionally, a woman who temporarily stopped ART and has started again during the same pregnancy should not be counted as new on treatment
- The number of HIV-positive pregnant women already on ART at beginning of pregnancy: May be counted even if ART is continuing to be received at another facility. For example, a woman who is already on treatment becomes pregnant and enrolls in ANC/PMTCT because she is HIV-positive but is continuing to receive her ART at a nearby treatment clinic should be counted within this disaggregate. However, if a woman was initiated on ART at another facility during this pregnancy and then transfers-in to the ANC site, she should not be counted (since she was already counted at the first ANC site for this pregnancy).
Auto-Calculated indicator in DATIM, sum of: 1) New on life-long ART, 2) Already on life- long ART at the beginning of the current pregnancy
Collected as part of PMTCT_STAT. Calculated indicator in DATIM, sum of: 1) New Positives, 2) Known Positive at entry (see PMTCT_STAT, Disaggregate Group Positivity Status for more details)
Indicator changes (MER 2.0 v2.3 to v2.4): None
PEPFAR-support definition:
Standard definition of DSD and TA-SDI used.
Provision of key staff or commodities for PMTCT include: commodities such as test kits, ARVs, lab commodities, or funding for salaries of health care workers.
Ongoing support for PMTCT service delivery improvement includes: training of PMTCT service providers, clinical mentoring and supportive supervision of PTMCT service sites, infrastructure/renovation of facilities, support for PMTCT service data collection, reporting, data quality, QI/QA of PMTCT services support, ARV consumption forecasting and supply management, support of lab clinical monitoring of patients, supporting patient follow- up/retention, support of mother mentoring programs.
Guiding narrative questions:
- Provide context for low PMTCT_ART coverage (PMTCT_ART / PMTCT_STAT_POS = ART coverage) by geographic area or partner/implementing mechanism, including any planned activities/remedial actions.
- Describe activities related to ensuring retention through the breastfeeding period. If additional data available in country, describe retention rates or rates of LTFU among pregnant women continuing or starting ART as of ANC1.
- Explain any differences in PMTCT_ART coverage among newly identified HIV positive women initiating ART compared to known positives already on ART.
Related Indicators