(LAB_PTCQI) Number of PEPFAR-supported laboratory-based testing and/or Point-of-Care Testing (POCT) sites engaged in continuous quality Improvement (CQI) and proficiency testing (PT) activities
Export Indicator
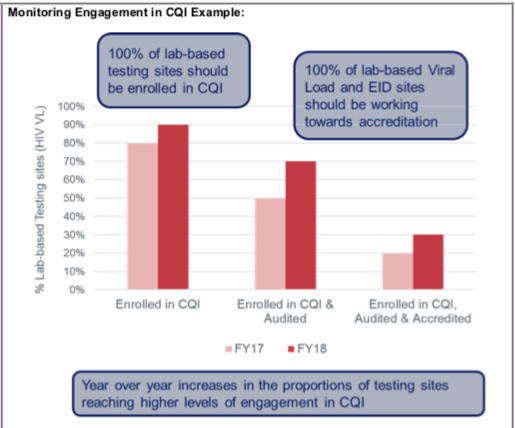
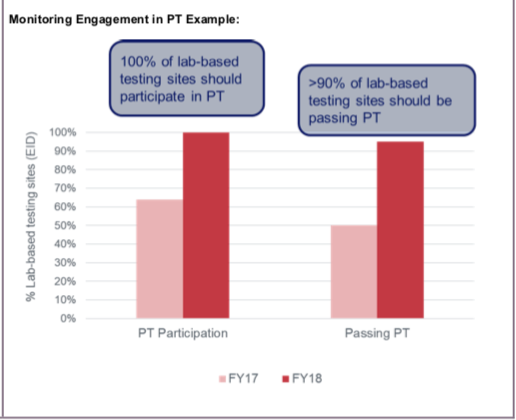
- Monitoring Engagement in CQI and PT: CQI and PT programs are critical to ensure efficient and quality assured laboratory testing. Monitoring testing sites’ levels of engagement in CQI and PT enables the identification of facilities, geographic areas, and implementing partners that may benefit from additional support related to laboratory quality. Engagement in CQI and PT may also be used to monitor progress over time (e.g., progress toward laboratory accreditation) and maintenance of quality assured laboratory testing.
- Recommendations for engagement in CQI and PT are outlined below. Implementing partners reporting data that do not meet these recommendations should be prepared to provide detailed explanations and action plans.
- 100% of laboratory-based testing sites participating in CQI and PT.
- 100% of HIV Viral Load and IVT/EID laboratory-based testing sites working towards accreditation.
- >70% of POCT sites, particularly HIV serology/diagnostic testing sites, participating in CQI and PT; with the goal of all POCT sites participating in CQI and PT.
- Year-over-year increases in the proportions of testing sites achieving higher levels of engagement in CQI (e.g., an increase in the proportion of accredited testing sites as compared to the previous year). Once saturation is achieved, it is critical that this indicator be used to monitor maintenance of CQI and PT programs.
- >90% of testing sites that conduct a test passing PT for that testing category.
- Recommendations for engagement in CQI and PT are outlined below. Implementing partners reporting data that do not meet these recommendations should be prepared to provide detailed explanations and action plans.
- Providing Context for Testing Results: Levels of engagement in CQI and PT may be used to provide context for testing results at the facility, SNU, or OU levels. Testing results reported in an SNU where a low percentage of testing sites are engaged in CQI, for example, may infer a lower degree of confidence than if the SNU had a high percentage of testing sites engaged in CQI. Please note that enrollment and achievement in CQI and PT programs are proxy indicators for laboratory quality and provide an indication of quality practices rather than a direct measurement of testing quality at the site
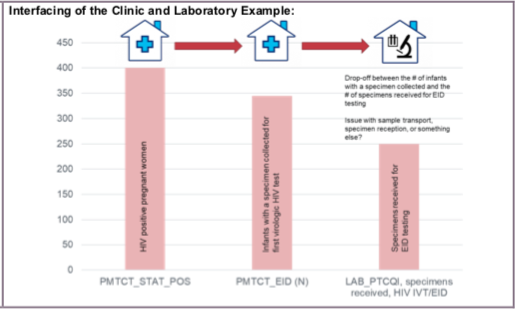
- Monitoring Availability of Laboratory Services: The number of specimens received for each testing category assesses the extent to which PEPFAR-supported laboratories and/or POCT sites are maintaining or expanding laboratory services. The number of specimens received may also be used to monitor the capacity of testing sites and scale- up efforts over time.
- Assessing the Clinic-Lab Interface: The number of specimens received for testing may be used in conjunction with other indicators to monitor the clinic-lab interface.
- Number of PEPFAR-supported laboratory-based testing and/or Point-of- Care Testing sites engaged in CQI activities.
- Number of PEPFAR-supported laboratory-based testing and/or Point-of- Care Testing sites engaged in PT activities.
- Number of specimens received for testing at all PEPFAR-supported laboratory- based testing and/or Point-of-Care Testing sites within a testing category.
N/A
Collect data for the LAB_PTCQI indicator, both laboratory and POCT, at facilities with PEPFAR-supported laboratories or POCT sites. A PEPFAR-supported laboratory or testing site is defined as a facility that receives direct service delivery (DSD) or technical assistance for service delivery improvement (TA-SDI) from PEPFAR, is the recipient of specimens from PEPFAR-supported clinics, and/or receives proficiency testing panels via PEPFAR support. See definitions for ‘laboratory’ and ‘POCT site’ below.
How many laboratory-based testing sites are in the facility?
A facility may have one laboratory-based testing site (e.g., HIV Viral Load laboratory-based testing site), multiple laboratory-based testing sites with different testing categories (e.g., HIV Serology/Diagnostic and HIV Viral Load laboratory-based testing sites), and/or multiple laboratory-based testing sites with the same testing category (e.g., Two HIV Viral Load laboratory-based testing sites - each under a distinct entity/department within the facility).
How many POCT sites are in the facility?
A facility may have one POCT site (e.g., HIV Rapid Test POCT site), multiple POCT sites with different testing categories (e.g., HIV Rapid Test POCT site and CD4 POCT site), and/or multiple POCT sites with the same testing category (e.g., Two HIV Serology/Diagnostic test POCT sites – one associated with the PMTCT program and the other associated with the TB program).
Where can data for this indicator be found?
Data on engagement in CQI and PT can be obtained from program records of PEPFAR- funded partners. Additionally, laboratory-based testing and POCT site-level documentation can be used to assess CQI engagement and PT results. Data on the number of specimens received for testing can be obtained from specimen registers/log books and/or laboratory information systems (LIS).
How are data interpreted and reported (Laboratory-Based Testing)?
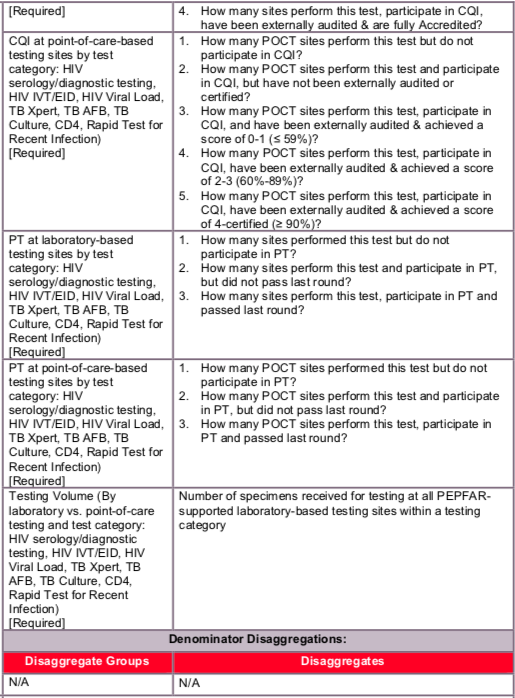
Identify the level of engagement in CQI activities for each laboratory-based testing site by choosing one of the following:
- Performs this test but does not participate in CQI (see definition of ‘CQI participation’ below).
- Performs this test and participates in CQI but has not been externally audited (see definition of ‘external audit’ below).
- Performs this test, participates in CQI, and has been externally audited, but does not meet full accreditation standards (see definition of ‘accreditation’ below).
- Performs this test, participates in CQI, has been externally audited, and is fully accredited.
Identify the level of engagement in PT activities for each laboratory-based testing site by choosing one of the following:
- Performs this test but does not participate in PT (see definition of ‘PT participation’ below).
- Performs this test, participates in PT, but did not pass the last round (see definition of ‘passing PT’ below).
- Performs this test, participates in PT, and passed the last round.
Sum the number of specimens received for testing at all laboratory-based testing sites within a testing category. See definition for ‘specimens received for testing’.
How are data interpreted and reported (Point-of-Care Testing)?
Identify the level of engagement in CQI activities for each POCT site by choosing one of the following:
- Performs this test but does not participate in CQI.
- Performs this test and participates in CQI but has not been externally audited.
- Performs this test, participates in CQI, has been externally audited, and achieved a score of 0-1 (≤ 59%).
- Performs this test, participates in CQI, has been externally audited, and achieved a score of 2-3 (60%-89%).
- Performs this test, participates in CQI, has been externally audited, and achieved a score of 4-certified (≥ 90%).
Identify the level of engagement in PT activities for each POCT site by choosing one of the following:
- Performs this test but does not participate in PT (see definition of ‘PT participation’ below).
- Performs this test, participates in PT, but did not pass the last round (see definition of ‘passing PT’ below).
- Performs this test, participates in PT, and passed the last round.
Sum the number of specimens received for testing at all POCT sites within a testing category. See definition for ‘specimens received for testing’.
DEFINITIONS (LABORATORY-BASED TESTING SITES):
Laboratory:
A. Having dedicated physical laboratory infrastructure
B. Having dedicated trained laboratory professionals performing testing
C. Conducting laboratory testing in one or more of the following areas:
a. Diagnosis of HIV infection with rapid test kits, EIA, WB or other molecular methods
b. Infant Virologic Testing / Early Infant Diagnosis (IVT/EID)
c. HIV viral load
d. TB diagnostics: Xpert, AFB, or culture
e. CD4 testing
f. Rapid Test for Recent Infection
Note: If a point-of-care assay (such as a rapid diagnostic test or Pima CD4) is performed at a laboratory-based testing site, as defined above, data should be reported in the laboratory portion of the indicator LAB_PTCQI indicator.
Laboratory-based testing site:
A point within a facility (with a PEPFAR-supported laboratory) that performs one of the tests defined in the testing categories within a laboratory.
Blood centers/banks:
Perform any service involved in blood donor recruitment, blood and plasma collection, testing, processing, storage, and distribution of blood and blood products. Stand-alone blood center/banks conducting testing such as screening and/or cross-matching are considered laboratories for this indicator.
CQI Participation:
CQI activities implement, improve, or maintain a Quality Management System (QMS). A functioning QMS is essential to provide accurate and reliable results with safety, efficiency, monitoring, and accountability throughout the testing process.
A laboratory-based testing site is counted as participating in CQI if they are engaged in activities within the testing category that are supported by a locally, nationally, regionally or internationally recognized CQI or accreditation preparedness program.
Examples of recognized programs:
A. Strengthening Laboratory Management Towards Accreditation (SLMTA)
B. Other established programs that utilize an auditing process such as WHO AFRO Stepwise Laboratory Quality Improvement Process Towards accreditation (SLIPTA) stepwise processes or CDC/PAHO Caribbean Laboratory Quality Management System Stepwise Improvement Process towards Accreditation (CDC/PAHO LQMS-SIP).
C. Locally-recognized basic laboratory quality management system programs
D. Locally-recognized laboratory mentorship programs
E. Participation in laboratory accreditation programs based on recognized laboratory standards such as African Society for Blood Transfusion (AfSBT), College of American Pathologists (CAP), or International Organization for Standardization (ISO).
External Audit:
Refers to a documented assessment conducted by a qualified external auditor. External audits can either be those for accreditation or those to assess readiness for accreditation such as WHO AFRO Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) and CDC/PAHO Caribbean Laboratory Quality Management System Stepwise Improvement Process towards Accreditation (CDC/PAHO LQMS-SIP). Internal assessments and audits, including those conducted as part of a training program curriculum; do not count towards this indicator.
Accreditation:
Refers to accreditation by a national, regional or internationally recognized accreditation body, such as College of American Pathologists (CAP), International Organization for Standardization (ISO) accreditation programs, regional accreditation bodies such as the South African National Accreditation System (SANAS), African Society for Blood Transfusion (AfSBT), or other approved accreditation organizations. A laboratory-based testing site is assessed by a standardized set of criteria defined by an acceptable national, regional, or international organization. Accreditation certificates are a formal recognition that a laboratory is competent to perform clinical testing. Laboratory-based testing site accreditation status must be current.
PT Participation:
Defined as enrollment/participation in a local, national, regional, and/or international external quality assurance or proficiency testing program at any time during the reporting period.
Passing PT:
A laboratory-based testing site is counted as passing PT if the last scheduled and completed PT panel was received within the reporting period and was scored as acceptable, successful, or satisfactory by the PT provider. Be aware that scoring systems between PT providers and across test categories may differ. All testing sites that are enrolled in PT should receive a score from the PT provider for each round of PT that is distributed, regardless of whether or not the site reported results.
Specimen received for testing:
A specimen is received for testing if its arrival at the laboratory-based testing site was recorded in a register/log book and/or LIS within the reporting timeframe. A specimen received for testing may or may not have been tested/analyzed.
DEFINITIONS (POINT-OF-CARE TESTING SITES):
POCT site:
A. The site performs testing near or at the place of interaction with the patient/client.
B. The site performs testing in an environment which does not have a formal laboratory infrastructure.
C. Testing at the POCT site is performed by healthcare workers who may not be laboratorians.
D. Conducting POCT in one or more of the following areas:
a. HIV rapid test
b. Infant Virologic Testing / Early Infant Diagnosis (IVT/EID)
c. HIV viral load
d. TB diagnostics: Xpert or AFB
e. CD4 testing
f. Rapid Test for Recent Infection
Notes: Sites conducting HIV rapid testing are considered POCT unless the testing is conducted in a laboratory (see definition of laboratory) by laboratorians. A laboratory-based testing site and POCT site may both be present at a facility. If a point-of-care assay (such as an HIV rapid test or Pima CD4) is performed at a laboratory-based testing site, CQI and PT data should be reported in the laboratory portion of the indicator (LAB_PTCQI (Laboratory)). LAB_PTCQI reporting only applies to facility-based testing. Data on CQI engagement, PT participation, or the number of specimens received for HIV rapid testing (or other POCT) that is conducted outside of a designated health facility (e.g., at a community-level service delivery point) should not be reported for LAB_PTCQI.
CQI Participation:
A POCT site is counted as participating in CQI if they are engaged in activities within the defined test category that are supported by a locally, nationally, regionally or internationally recognized CQI or certification preparedness program.
Examples of POCT CQI programs:
A. Rapid Testing Continuous Quality Improvement (RT-CQI)
B. Other established programs that utilize WHO/CDC Stepwise Process for Improving the Quality of HIV rapid testing (SPI-RT) or the WHO/CDC Stepwise process for Improving the Quality of HIV-Related Point-of-Care-Testing (SPI-POCT) Checklists to audit the POCT sites.
C. Locally-recognized basic quality management system programs
D. Locally-recognized basic quality management system programs
External Audit or Certification:
Refers to a documented assessment conducted by a qualified external auditor. These audits include those for national POCT site certification or for a stepwise quality improvement approaches such as the WHO/CDC Stepwise Process for Improving the Quality of HIV rapid testing (SPI-RT) or the WHO/CDC Stepwise process for Improving the Quality of HIV-Related Point-of-Care-Testing (SPI-POCT) Checklists. Internal assessments and audits, including those conducted as part of a training program curriculum; do not count towards this indicator.
PT Participation:
Defined as enrollment/participation in a local, national, regional, and/or international external quality assurance or proficiency testing program within the reporting period.
Passing PT:
A POCT site is counted as passing PT if the last scheduled and completed PT panel was received within the reporting period and scored as acceptable, successful, or satisfactory by the PT provider (see ‘Passing PT’ under laboratory testing for more information). For HIV rapid testing, if multiple testers at a POCT site participate in the same round of PT, >90% of testers must receive a passing PT score of 100% for the POCT site to be reported as passing PT. If the HIV rapid testing PT program provides one PT panel for the site (as opposed to one PT panel for each tester), the POCT site must have a PT score of 100% to be reported as passing PT.
Specimen received for testing:
A specimen is received for testing if its arrival at the POCT site was recorded in a register/log book and/or LIS within the reporting timeframe. A specimen received for testing may or may not have been tested/analyzed.
How to review for data quality:
The total numerator is automatically summed across the CQI and PT data elements for each laboratory-based testing category. This sum should equal the total number of laboratory-based testing and/or POCT sites for in each testing category at the facility and should be the same between the CQI and PT sections.
Reporting Level: Facility

Disaggregate descriptions & definitions:
- For both CQI and PT disaggregate groups, testing category disaggregations are only applicable if specific test category is performed by the laboratory.
- The most recent PT panel with a score must be satisfactory/acceptable/successful to be counted as a passing score.
The numerator is generated by counting the number of PEPFAR-supported laboratory- based testing and point-of-care testing sites for each testing category by their level of engagement in CQI and PT activities; and the number of specimens received for testing at laboratory-based testing and point- of-care testing sites within each testing category.
Indicator changes (MER 2.0 v2.3 to v2.4):
- Laboratory and point-of-care testing site categories updated to include “rapid test for recent infection”
- Laboratory and point-of-care testing site categories updated to remove “other”
PEPFAR Support definition: Standard definition of DSD and TA-SDI used.
Guiding narrative questions:
- In the narrative, please define how the specimen volume was counted (i.e., specimen log, LIS, etc.).
Data Visualization & Use Examples: